A 20-g sample of steam. Which one of the following phrases is an example of.

Thermodynamics Why Do Some Substances Undergo Sublimation While Others Do Not Physics Stack Exchange
When a substance undergoes fusion it freezes.

. Using the heat of vaporization of water 540 calg the specific heat of water and the heat of fusion of water 80 calg find the total heat in calories that are released when a 250 g sample. In this case when heat is applied to the system its. The process of fusion of substances takes place during the conversion of a solid substance into a liquid.
When a substance boils it undergoes a process known as. Complete the following statement. When a system undergoes phase changes such solid to liquid or liquid to gas the temperature of the system will remain constant.
Promise audit to gas see it. When a substance undergoes fusion it A freezes. When a substance undergoes fusion it vaporizes.
This occurs when the internal energy of the solid increases typically by the. When a substance undergoes fusion it a Evaporates O b Freezes O c. Melting or fusion is a physical process that results in the phase transition of a substance from a solid to a liquid.
So in this question they asked when a substance undergoes a chemical change it is salvaged to that A it liquefies be it changes. Hope i helped Is heat the. When a substance melts it undergoes a process called.
When a substance undergoesfusionit a freezes. Complete the following statement. Vaporization of a sample of liquid is a phase transition from the liquid phase to the gas phase.
Complete the following statement. Complete the following statement. Heat is added to a substance but its temperature does not rise.
Melting or fusion is a physical process that results in the phase transition of a substance from a solid to a liquid. THIS SET IS OFTEN IN FOLDERS WITH. Evaporates Posted 2 months ago Q.
There are two types of vaporization. The phase change of Solid to Gas is known as. The term heat most.
Questo 2011 polnt Saved Complete the following statement. A Which one of the following phrases is an example. What is the change in entropy of 1 kg of water when it is heated from.
There are two types of vaporization. When a substance undergoesfusionit a freezes. Vaporization of a sample of liquid is a phase transition from the liquid phase to the gas phase.
When a substance undergoes fusion it. This occurs when the internal energy of the solid increases typically by the. When a substance boils it undergoes a process known as.
When a pure substance undergoes a chemical change it is no longer that same substance. When a substance boils it undergoes a process known as. A chemical change changes the identity of the substance.
When a substance undergoes fusion it a freezes. Which one of the following statements provides the best explanation for this observation. Complete the following statement.
Which one of the following phrases. 100 4 ratings Transcribed image text.
Solved If The Fusion Of Two Unknown Atoms Results In A Particle With 0 0000043 Kg Less Mass Than The Sum Of The Masses Of The Two Unknown Atoms How Much Energy Is
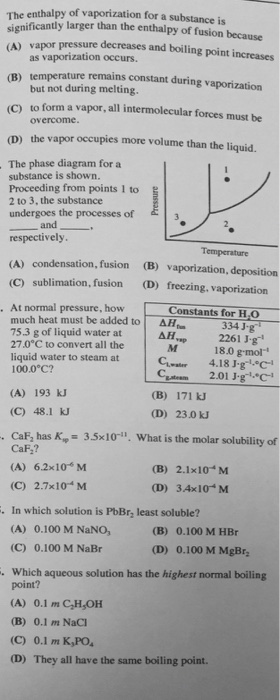
Solved The Enthalpy Of Vaporization For A Substance Is Chegg Com

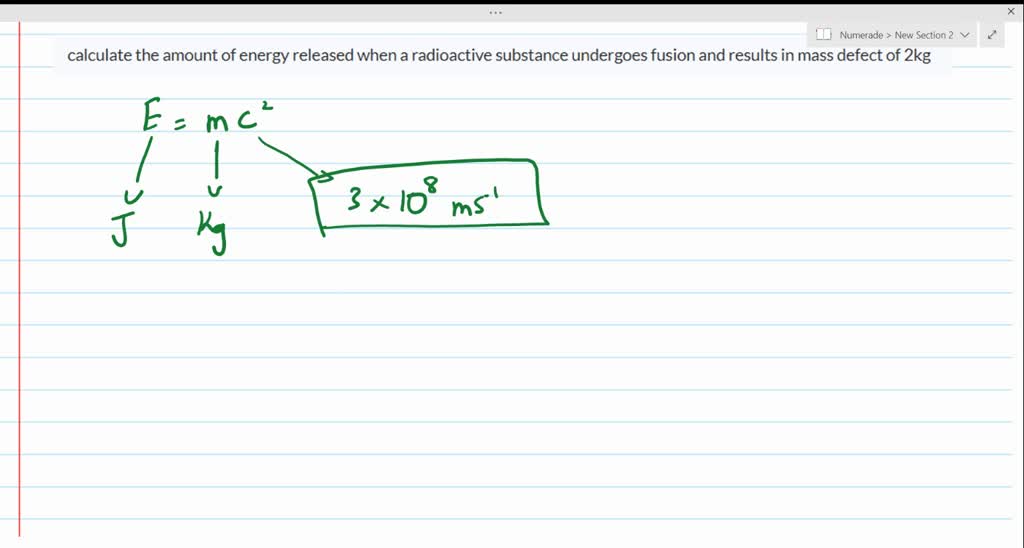
Calculate The Amount Of Energy Released When A Radioactive Substance Undergoes Fusion And Results In A

Defining Temperature Temperature Is A Measure Of The Average Kinetic Energy Of The Particles In A Substance Adding Or Removing Energy Usually Changes Ppt Download
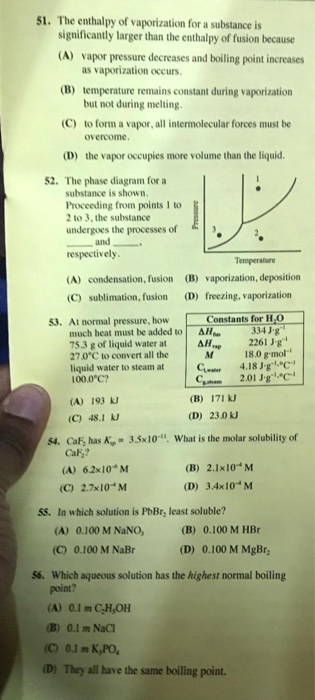
Solved 51 The Enthalpy Of Vaporization For A Substance Is Chegg Com

Do Now Hand In Specific Heat Lab Answer The Following Question On Do Now Sheet 56 Grams Of Hot Copper Are Added To 140 Grams Of Water In A 92 G

Advanced Chemistry Ms Grobsky Enthalpies Of Reactions And Hess Law Ppt Download
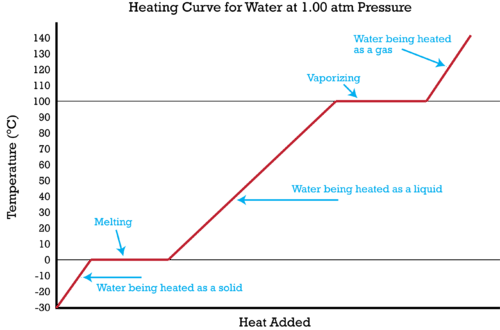
Multi Step Problems With Changes Of State Ck 12 Foundation

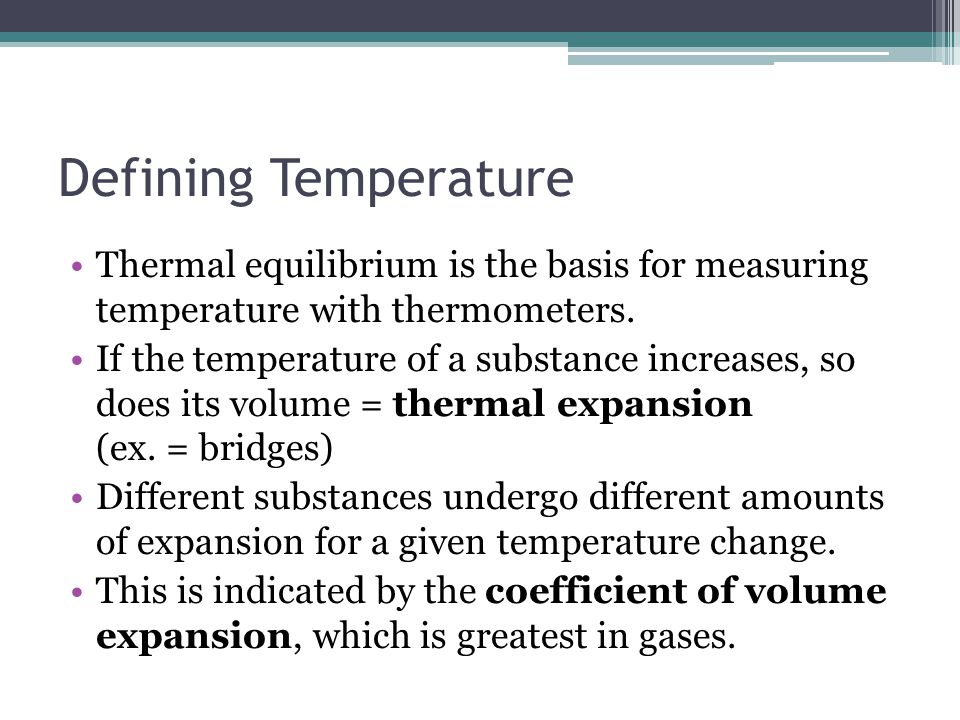
Chapter 9 Preview Objectives Defining Temperature Thermal Equilibrium Ppt Download

Heat And Temperature Let S Review According To The Kinetic Theory Of Matter All Matter Is Made Up Of Tiny Particles Called Atoms Or Molecules Ppt Download

A Substance Is Heated At A Constant Rate And Its Temperature Is Taken Every Minute During The Heating The Substance Undergoes One Change Of State The Results Are Shown In The Graph

Chapter 20 Heat Specific Heat Internal Energy First Law Of Thermodynamics Part 3 Thermodynamics Chapter 20 Heat And The First Law Of Thermodynamics Reading Ppt Download

Solids Liquids Energy Ppt Download

Phy 102 Fundamentals Of Physics Ii Chapter 17 States Of Matter Lecture Notes Ppt Download

Do Now Hand In Specific Heat Lab Answer The Following Question On Do Now Sheet 56 Grams Of Hot Copper Are Added To 140 Grams Of Water In A 92 G

Lab 12 Heat Energy And Temperature This Is It Today We Are Going To Measure The Specific Heat Of An Unknown Metal Important Terms Temperature T Ppt Download
Unit 4 Thermodynamics Chapters 9 And Ppt Video Online Download

Energy Transformations Ppt Download
